Abstract
BACKGROUND AND AIM: Hemolysis, inflammation and coagulopathy associated with sickle cell disease (SCD) can lead to pulmonary hypertension. More than 15% of adult patients with SCD are affected by pulmonary hypertension as measured by a tricuspid regurgitation velocity (TRV) ≥ 2.9 m/sec (Nouraie, M. et al., Validation of a composite vascular high-risk profile for adult patients with sickle cell disease. American Journal of Hematology, 94:E312-E314. 2019). Moreover, sickle cell disease patients with pulmonary hypertension have a much higher mortality risk than those without pulmonary hypertension. There is little knowledge on the proteomic profile of pulmonary hypertension in SCD. Our aim was to conduct a proof-of-concept study to explore targeted serum protein biomarkers that are differentially expressed in SCD patients with elevated tricuspid regurgitation velocities and explore their corresponding pathways.
MATERIAL AND METHODS: Data from the Walk-PHaSST study was used for this study. The Walk-PHaSST was a multicenter study to assess the effect of sildenafil for SCD patients with pulmonary hypertension (Gladwin, M. T. et al., Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS One, 9:e99489. 2014). In the screening phase of the study, 720 patients with SCD were recruited. Study participants underwent clinical and lab examinations, as well as an echocardiography. We selected two groups of patients: one group of patients with a TRV≤2.6 m/sec and another with TRV≥2.9 m/sec (N =35 in each). The serum concentration of 92 protein biomarkers was measured using an OLINK cardiovascular panel. This panel assess a range of biological process including inflammatory response, angiogenesis, cell adhesion, coagulation, and response to hypoxia. T-tests were performed between the aforementioned groups for each of the serum biomarkers. The Hochberg method was used to calculate the false discovery rate. A volcano plot was created using the Bonferroni correction. Finally, we tested the correlation of the differentially expressed biomarkers with clinical variables measured in blood samples of the participants. These variables include white blood cell (WBC), neutrophil count, thrombospondin-1 (TSP as measure of coagulation), PIGF and VEGF (growth factors involved in angiogenesis), and NT-proBNP (elevated in pulmonary hypertension).
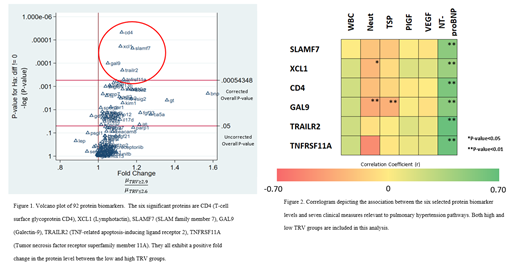
RESULTS: Using a false discovery rate of 0.01, we discovered 14 significantly expressed proteins. Six of them passed a Bonferroni corrected overall critical p value < 0.00054 (Figure 1). These included T-cell surface glycoprotein (CD4), lymphotactin (XCL1), SLAM family member 7 (SLAMF7), galectin-9 (GAL9), TNF-related apoptosis-inducing ligand receptor 2 (TRAILR2), and tumor necrosis factor receptor superfamily member 11A (TNFRSF11A). We observed up to a 1.2-fold increase in these 6 protein biomarker levels in the high TRV groups.
The correlogram (Figure 2) indicated that there is a slightly positive correlation between WBC and CD4, GAL9, TRAILR2, and TNFRSF11A (r > 0.20). For neutrophil count, we observed significantly negative correlation with selected markers including TNFRSF11A levels (r = -0.51), GAL9 (r = -0.35), XCL1 (r = -0.27), SLAMF7 (r = - 0.21), and CD4 (r < - 0.24). A significantly negative correlation between TSP and GAL9 levels (r = -0.31) was also observed. Finally, we observed a strong, positive correlation between all proteins and serum NT-proBNP levels (r > 0.44).
CONCLUSION: Circulatory protein markers of immune response and coagulation are highly expressed in SCD patients with elevated TRV. This provides evidence that these protein biomarkers have potential to be utilized as prognostic markers for pulmonary hypertension in patients with SCD.
Little: Biochip Labs: Patents & Royalties; Hemex Health, Inc.: Patents & Royalties. Gibbs: Acceleron Pharma: Consultancy, Other: lecture fees; Actelion: Consultancy, Other: lecture fees; Aerovate: Consultancy, Other: lecture fees; Bayer: Consultancy, Other: lecture fees; Compexia: Consultancy, Other: lecture fees; Janssen: Consultancy, Other: lecture fees; MSD: Consultancy, Other: lecture fees ; Pfizer: Consultancy, Other: lecture fees; United Therapeutics: Consultancy, Other: lecture fees. Gordeuk: Modus Therapeutics: Consultancy; Novartis: Research Funding; Incyte: Research Funding; Emmaus: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; CSL Behring: Consultancy. Nouraie: Phoenicia BioScience Inc.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal